Design and build regulatory. A cleanroom or clean room is a facility ordinarily utilized as a part of specialized industrial production or scientific research including the manufacture of pharmaceutical items integrated circuits crt lcd oled and microled displays.
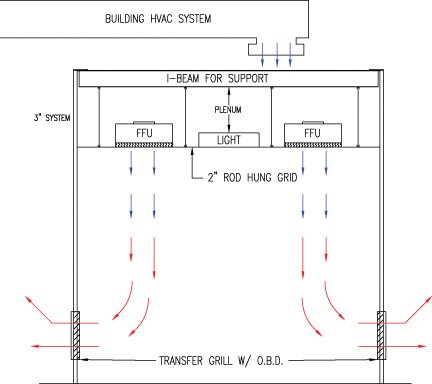
Clean Room Design Considerations Technique
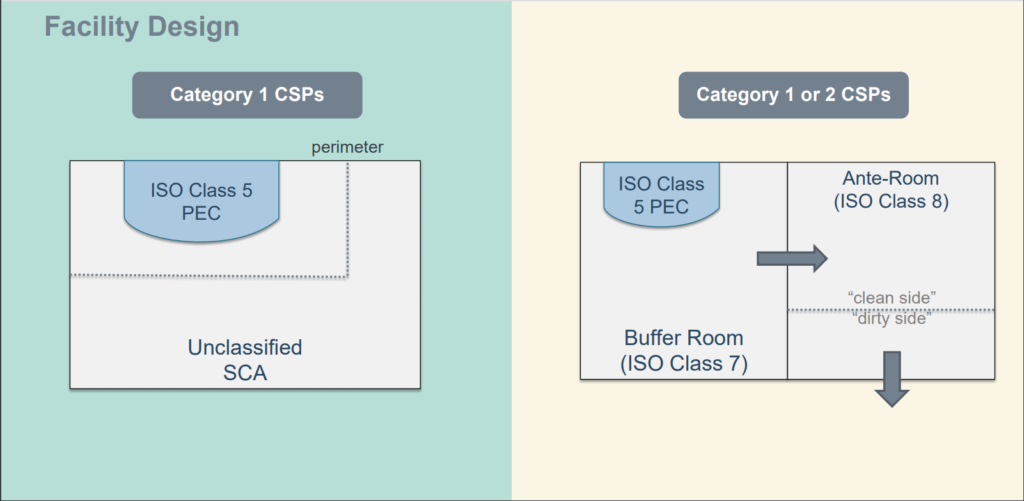
For aseptic preparation and filling this is the background environment for grade a zone.

Aseptic room design. Most facilities benefit from contacting a controlled environment specialist who can identify. In this video here is explanation about specifications factors consideration for aseptic area designing and aseptic area. Snehal patel assistant professor sumandeep vidyapeeth vadodara gujarat india.
A clean room gmp cleanroom in my mind are a combination of engineering design fabrication finish and operational controls control strategy that are required to convert a normal room to a clean room. Space requirements volume of operators aseptic workflows material choice and even aesthetics all factor into design considerations. The maintenance of laminarity should be demonstrated and validated.
Aseptic area is an extremely important work place for sterile preparation. Compounding of aseptic manipulations must be performed in an iso class 5 or fed 209e class 100 environment. Basic clean room requirements designs for gmp clean rooms what is a clean room.
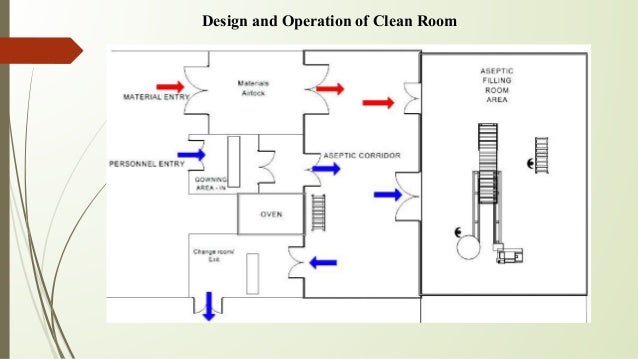
Design the room to sustain a contamination free environment. There is no mathematical formula when determining the features surfaces and hvac requirements best suited for your application. Aseptic materials and room design options help companies prepare to perform important medical or biopharmaceutical operations as well as others requiring an easy to sterilize environment.
Value at the working position in open clean room applications. For the surrounding areas it is recommended that the cleanliness and. A uni directional air flow and lower velocities may be used in closed isolators and glove boxes.
Building design construction and production facilities production of sterile products should be carried out in a clean environment with a limit for the environmental quality of microbial and dust particle. Design of aseptic area prepared by mr. You can get such a room in a few days and be ready for operation quickly while meeting regulatory guidelines.
Cleanrooms are designed to maintain extremely low levels of particulates such as dust airborne organisms or vaporized particles. Clean room design in sterile pharmaceutical manufacturing has an important role in minimizing the contamination. Read it in detail here.
China has implemented a new version of its good manufacturing practice gmp standard and has completely adopted eu gmp cleanliness standards and introduced the in operation classification. Designing facilities for aseptic filling. A gowning room has many variables.

Sterile Area Cleanroom Qualification Pharmaceutical Guidelines
Http Www Who Int Medicines Areas Quality Safety Quality Assurance Supplementarygmpheatingventilationairconditioningsystemsnonsterilepharmaceuticaldosageformstrs961annex5 Pdf Ua 1

China Aseptic Clean Room Portable For Pharmaceutical Industry
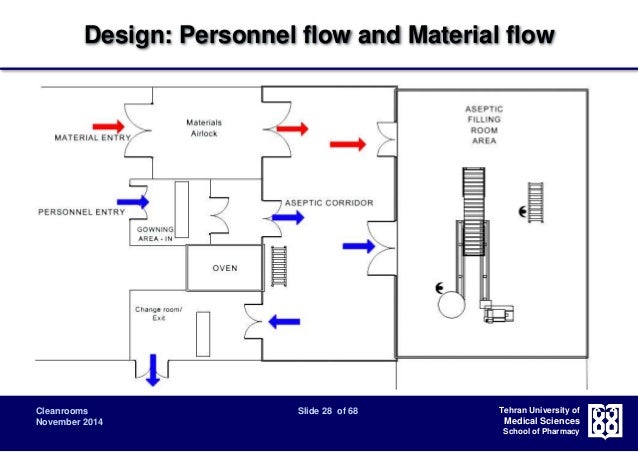
Cleanroom Classification Design And

New Tech Disrupts Traditional Aseptic Processing
Designing Flexible Aseptic Containment Systems Pharmaceutical
Aseptic Laboratory Requirements And Daily Management News

Usp 797 Clean Room Guidelines Standards For Sterile Compounding Labs
Aseptic Technic And Aseptic Room Authorstream
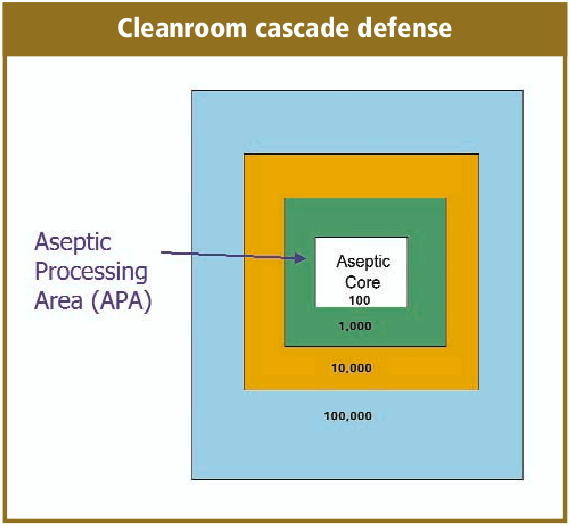
Overview Of Aseptic Fill Finish Manufacturing Biorealty Inc

Aseptic Packaging An Overview Sciencedirect Topics

Hvac Design Hvac Design In Pharmaceutical Facility

Cleanroom Technology Future Is Gloveless For Biopharma Aseptic
Case Studies Aberdeen Royal Infirmary Asu Envair Projects
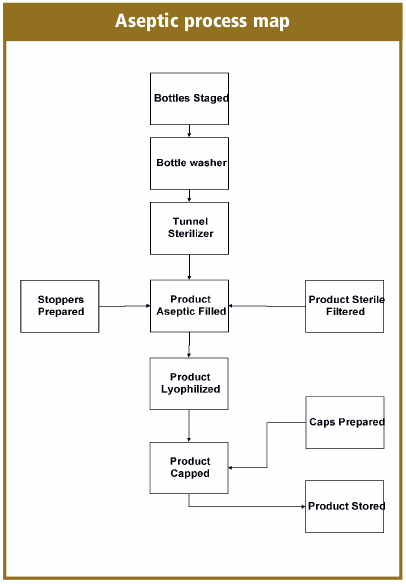
Overview Of Aseptic Fill Finish Manufacturing Biorealty Inc
Pharmacy Planning And Design Projects Aseptic Enclosures
Hospital Concept And Design Built In Operating Theatres Akcmed
Clean Room Design Considerations

Dispensing The Cyclotron Facility At The University Of Chicago




No comments:
Post a Comment